Single stem visceral debranching for complex aortic disease

| Available Online: | September, 2023 |
| Page: | 36-42 |
Author for correspondence:
Jean-Michel Davaine, MD, Ph.D
Sorbonne Université, Faculté de Médecine Campus Pitié-Salpêtrière, Paris, France
Tel: +33(0)783475673
Email: davainej@yahoo.fr
doi: 10.59037/hjves.v5i2.30
ISSN: 2732-7175 / 2023 Hellenic Society of Vascular and Endovascular Surgery
Published by Rotonda Publications
All rights reserved. https://www.heljves.com
J.M. Davaine1,2,*, J. Jayet2,*, L. Oiknine2, G. Martin2, T. Couture2, D. Verscheure2, J. Gaudric1,2, L. Chiche1,2, F. Koskas1,2
1Sorbonne Université, Faculté de Médecine Campus Pitié-Salpêtrière, Paris, France
2Vascular surgery department, University hospital Pitié-Salpêtrière, Paris, France
*Contributed equally to this work and are to be considered as co-first authors.
Keywords: Hybrid surgery, visceral vessel debranching, TEVAR, aortic dissection, thoracoabdominal aortic aneurysm
Abstract
Full Text
References
Images
Abstract
What this paper adds
Treatment of complex aortic diseases including thoracoabdominal aortic aneurysms and aortic dissection is highly challenging. Hybrid repair may be useful in some situations wherein anatomy, the need of emergent repair or patient comorbidities preclude the use of total endovascular or direct open reconstruction. This paper details an original hybrid repair in which a single branch is used to reroute all visceral vessels.
Abstract:
Objective: Hybrid treatment of complex aortic disease has been described with various techniques of retrograde visceral bypass. The use of a single branch to revascularize all renal and visceral vessels may be less cumbersome than multiple synthetic branches and may seems to be efficient in terms of patency.
Methods: We retrospectively included 15 patients between 2013 and 2021. Indication was aortic dissection (AD) (type A, acute or chronic type B), thoracoabdominal aortic aneurysms (TAA), visceral occlusive disease. Surgery consisted in median laparotomy, visceral vessel debranching from native aorta or from an aortic graft. In case of AD, surgical fenestration was performed. Additional TEVAR completed the treatment when indicated, during the same procedure or later on
Results: Mean age was 60 years. 9 (60%) patients were treated for AD, 3 (20%) for TAA, 3 (20%) for occlusive disease. A total of 65 target
vessels were debranched through the single stem retrograde vascular graft (SSRVG) technique. Aortic surgical fenestrations were performed in 8 cases and TEVAR in 4 cases. In the postoperative course, 3 TAA patients died,7 patients developed renal insufficiency (47%), 4 patients presented pneumonia (27%) and 3 colonic ischemia (20%). After a mean follow up of 21 months, all vessels (but 2 IMAs) were patent and no endoleak was noted.
Conclusion: SSRVG technique offers a feasible and safe solution in various complex aortic diseases. The use of a single graft makes the technique straightforward by reducing the volume of multiple branch assembly in the retroperitoneal space with satisfying patency rates. Further studies with larger patient sample size and longer follow up are needed to elucidated the efficacy and durability of the technique.
Full Text
INTRODUCTION
Treatment of complex aortic disease such as thoracoabdominal aortic aneurysm (TAA) or aortic dissection (AD) is a challenge for both patients and surgeons and the best therapeutic option is still subject to controversies. Open repair remains a challenge and even with improvements like distal perfusion and spinal protection, only fit patients with limited comorbidties can undergo such invasive procedure. More and more TAA and AD cases become amenable to a totally endovascular repair due to constant progress of devices and skills 1,2. Nonetheless, uncertainty of long-term results, limits in technical feasibility and availability of devices still restrict its wide applicability. As a result, there are situations wherein total open surgery is considered a great surgical “insult” and total endovascular repair is not an option. For those patients can hybrid repair, combining both open and endovascular techniques, offer an appealing alternative. Several reports been published since the nineties underlining the role of debranching of the supra aortic trunks and a tubular stent-graft on the treatment of lesions of the aortic arch using 3,4 . The same strategy may be used for the treatment of abdominal or thoracoabdominal lesions. Visceral vessels are bypassed first, paving the way for a synchronous or metachronous exclusion of the aortic lesion by a stent-graft 5,6. The stemming of visceral debranching from the infrarenal aorta avoids the need for proximal aortic clamping, thoracotomy, and limits the duration of and end-organ ischemia. However various techniques of debranching stemming either from the infrarenal aorta or the iliac arteries have been described, they did not reach the same level of popularity as that of arch debranching 5 . This lack of success may be due to several reasons for technical failure including a poor donor artery, the use of multiple grafts in an “octopus” configuration and suboptimal choice of the best tunnels for retrograde visceral grafts. With the disappearing of the skills in open abdominal vascular surgery among (young) vascular surgeons we have found useful to herein report our original technique of abdominal visceral debranching using a single stem retrograde visceral graft (SSRVG). Indications, principles, technical details and outcomes of patients are provided.
METHODS
Patients et indication
This is a single center retrospective study. Between 2013 and 2019, 168 patients who were treated for a visceral artery surgery were identified. Patients selection used the French common classification of medical procedure (CCAM) with a specific code, namely EDKA003: «remplacement d’une artère digestive par laparotomie». Decision to perform a hybrid visceral debranching rather than a total open or endovascular treatment resulted from a multidisciplinary consensus. All medical records were reviewed and patients treated with this particular single branch retrograde bypass technique were identified. A total of 15 patients were identified. Data were collected on an Excel spread-sheet on a password-protected computer, including demographics and clinical characteristics, type of the disease (TAA, AD, occlusive disease), emergency or elective cases, symptoms at presentation, preoperative workup, intraoperative data and postoperative course. Target vessel patency was evaluated on most recent postoperative CT scan. Given the nature of the study, a waiver was given by the Sorbonne University review board regarding informed consent of patient.
APPROACH
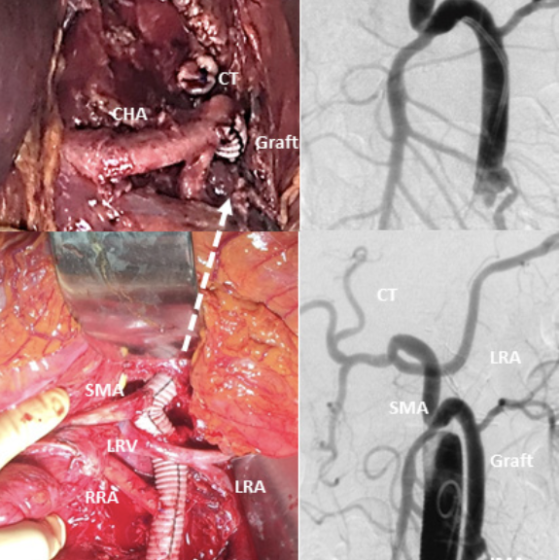
Patients are operated on under general anesthesia in a supine position with mild lordosis. A transperitoneal median laparotomy is performed in all cases. The retroperitoneum is firstly entered lateral to the duodenojejunal junction after section of the lesser mesenteric vein. The left renal vein (LRV) is mobilized using a large rubber tape, permitting exposure of the abdominal aorta proximal and distal from the renal ostia up to the iliac arteries. The right and left renal arteries (LRA and RRA) and inferior mesenteric artery (IMA) are then dissected distal to any ostial lesion and taped. The superior mesenteric artery (SMA) is exposed through a left lateral approach from its ostium to distal to any ostial lesion. Exposure of the SMA can be demanding while expose of its ostial segment deals with the felting of mesenteric plexus. Approach of the intramesenteric segment necessitates the section of the Treitz ligament. To get a full mobilization of the SMA one or both pancreaticoduodenal branches must be ligated. The celiac trunk (CT) is approached through a section of the lesser omentum. After the taping of the common hepatic and splenic arteries, the coronary gastric artery is ligated and the trunk is dissected from the celiac plexus, through the arcuate ligament and if necessary, the right crux of the diaphragm. A retropancreatic tunnel is quite easy to create at this step from the left lateral side of the suprarenal aorta to the left lateral side of the celiac trunk by severing the left crux of the diaphragm. In cases of increased fragility of aortic wall (i.g. aortic dissection) a too proximal approach of visceral arteries must be avoided.
Choice of the donor artery
Many failures of retrograde debranching derive from a bad choice for the donor artery like a diseased iliac artery. Our, therefore, best choice is an aorto-aortic, aorto-(bi)iliac or aor to-(bi)femoral prosthetic graft. In AD cases, such a choice offers the opportunity of creating an aortic fenestration.
Preperation of the graft
A custom graft is created by assembling a main graft (MG) with a retrograde visceral graft (RVG). The choice for the MG can be any polyester or PTFE graft but for bifurcations, we prefer asymmetric bifurcations in the aorto(bi) iliac setting (Albograft 18x10mm, 16x9mm or 14×8 Lemaitre Burlington Massachusetts USA). For the RVG, some resistance to kinking is needed, therefore a polyester- coated PTFE graft (Fusion 10mm or 8mm, Maquet Getinge Merrimack, USA) is chosen. An elliptic hole is created on the left lateral side of the body of the MG using a cautery. The hole is positioned two centimeters above the aortic bifurcation or the bifurcation of the graft in order to maximize the length of the MG proximal to the anastomosis with the RVG and subsequently securing an adequate distal landing zone for the thoracic stent graft. A beveled retrograde anastomosis is then made using a running polypropylene 5/0 suture end of the RVG to side of the MG.
Proximal anastomosis of the main graft
Systemic heparin (100 UI/Kg) is given prior to clamping. Sites of clamping and of anastomosis are carefully planned on preoperative imaging and by intraoperative palpation. Attempts at clamping or suturing diseased, thrombosed or calcified aortic segments must be avoided. Typically, the clamp is applied one centimeter caudal to the most distally arising renal artery. Although our technique is compatible with a side to end anastomosis, we prefer an end to end anastomosis. The infrarenal aorta is then sectioned transversally one to two centimeters distal to the clamp. The proximal aortic section is then anastomosed to the MG end to end using a running 4/0 or 3/0 polypropylene suture. If the MG is bifurcated, it is important to position its bifurcation approximately at the same level as that of the native aortic bifurcation. Doing so leaves a long possible prosthetic landing zone proximal to the arising of the RVG.
Proximal anastomosis whenever dealing with an aortic dissection
When dealing with AD, even if all visceral arteries are repaired using the RVG, there is a major concern regarding the flow to upper lumbar or intercostal arteries, which can be impaired with an eventual spinal injury. In these cases, open fenestration is a solution to preserve perfusion to both lumens. It is then important to leave two to three centimeters between the level of clamping and the proximal anastomosis. Dissected tissues are particularly fragile. Therefore, clamping must be gentle and progressive, under moderate hypotension and using clamps with rubber-protected jaws. After clamping, the proximal section of the aorta is exposed using “stay sutures”, allowing for a neat resection of the flap flush to the aortic wall and to the clamp. It is in those cases when it may be useful to clamp the suprarenal aorta in order to ease the resection of the flap. The proximal anastomosis is then sutured, preferably using a running polypropylene Teflon-felt-reinforced running suture.
Distal anastomosis to the aortic bifurcation or distal anastomoses to iliac or femoral arteries is/are then made to allow flow to the lower limbs and pelvis and provide optimal tension of the MG.
Visceral debranching and repair
Visceral debranching and repair is then performed in a sequential caudal to cephalad order: IMA if considered necessary, most distal renal artery, most proximal renal artery, SMA and CT. Except for the IMA, every visceral artery to be treated is ligated or clipped at its ostium and sectioned at its most healthy level. The distal stump is beveled if smaller than 4mm or to give a good direction and transposed side to end to a hole punched on the RVG using a running suture of 5/0 or 6/0 polypropylene. If the IMA is treated, it is transposed via a small Carrel button of adjacent aortic wall. The best anastomotic position for the IMA is on the left lateral aspect of RVG one centimeter above its anastomosis with the MG. The best position for the renal arteries is the ipsilateral aspect of the RVG behind the taped LRV. The RVG must therefore be tunneled behind the LRV. Anastomosis to the LRA is in general easier than to the RRA. For the RRA, especially if its level of arising from the aorta is close to the ostium of the SMA, it is practical to sever firstly the SMA to facilitate the access to the RRA. All anastomoses to the visceral arteries must be performed without tension. For this reason, it is important to free a sufficient length of the artery to allow for its coming to the RVG without tension. If this is not possible due to anatomical or pathological reasons, it is advisable to interpose a short segment of prosthetic graft of an adapted size between the target artery and the RVG. This is particularly important for the SMA, which is optimally transposed on the frontal aspect of the RVG, either caudal or well proximal of the crossing of the RVG by the LRV, in order to avoid the creation of a nutcracker syndrome. The RVG is then tunneled behind the pancreas and anastomosed end to end to the distal section of the CT. Flow to every target artery is then checked using a continuous sterile doppler probe. In fat or normally adipose patients After completion of the visceral debranching the RVG can be covered using the left mesocolon and the pre-aortic tissue, whereas a trans-mesocolic omentoplasty can often be necessary to secure the graft.
Postoperative course and follow up
All patients were admitted in ICU during the first postoperative days then cleared to the ward in the absence of a complication. Major neurological, respiratory, renal, cardiac and digestive adverse events occurring until discharge were collected. Patients had duplex and CTA before discharge. After discharge, patients were followed up at 1 month, 6 months and yearly thereafter, clinically, with duplex or CTA.
RESULTS
Intraoperative
From January 2015 to December 2019, fifteen patients were treated using this technique.
Mean age was 60 years old [38-78]. Other demographics and cardiovascular risk factors are given in Table I. Table II details indications and outcomes. The indication was an AD in 9 (60%) cases, a TAA in 3 (20%) cases, and mesenteric occlusive disease in 3 cases (20%). Nine patients had prior vascular interventions, two of whom including abdominal aorta replacement, providing a suitable MG at the time of debranching.
A total of 65 target arteries were debranched and repaired through a single-stemmed-RVG: 5 target arteries were treated in 8 (53%) cases, 4 in 4 (27%) cases and 3 in three (20%) cases. Aortic fenestration was performed in 8 (53%) cases. Four (27%) procedures included the insertion of a tubular aortic stent-graft, two in a single-stage operation and two in a second procedure. Mean operative time was 354±72 minutes.
Out of the four patients who received TEVAR, 3 had spinal fluid drainage. One, who was operated on an emergency setting and was under 2 antiplatelet agents, had no drainage and experienced secondary paraplegia.
Treated pathologies
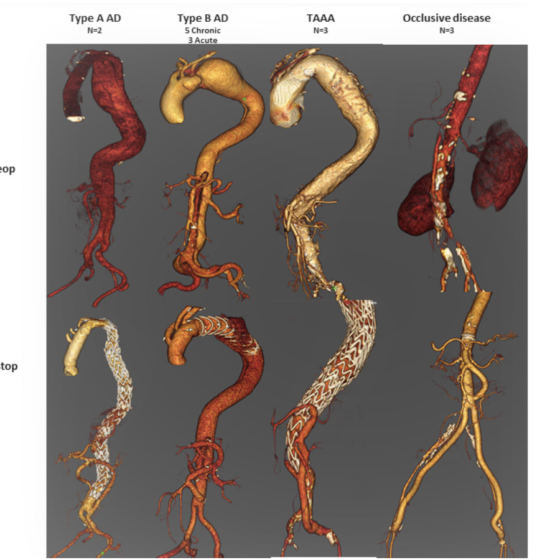
Type A aortic dissection: patients #1 and 2
These two patients had both open repair of their AD (Bentall procedure). The first patient developed a type 1 dissecting TAA during follow up. The second patient presented later on with a 45mm left common iliac artery dissecting aneurysm and all of the visceral arteries were dissected. His descending thoracic aorta was 42mm in diameter.
Chronic type B aortic dissection: patients #3-6
These patients were previously treated medically (#5), with TEVAR (#4) or open surgery (#3,6). Later on, they devel oped various degree of visceral/ renal malperfusion along with enlargement of their descending thoracic aorta or abdominal aorta requiring aneurysm exclusion and visceral/ renal vessels revascularization.
Acute type B aortic dissection: patients #7-9
Indication was persistent hypertension, visceral/ renal malperfusion and/ or rapid enlargement of their aorta.
Thoraco-abdominal aortic aneurysm: patients #10-12
These patients had TAA and were deemed unfit for total open repair, not amenable for total endovascular repair for anatomic reasons, or were treated in emergency.
Occlusive disease: patients #13-15
Patients in this group presented with chronic mesenteric ischemia and aorto-iliac occlusive disease and retrograde by-pass was favored over antegrade reconstruction.
Postoperative course
There were three (20%) postoperative deaths recorded. Two patients succumbed on the 2nd and 45th day due to multi-organ failure (MOF) whereas the third patient deceased on 51st postoperative day after discharge to a rehabilitation center due to aspiration associated pneumonia and respiratory failure. No death was related to RVG occlusion. Among survivors, acute renal insufficiency was the most frequent complication; 7 (47%) patients, two of which requiring temporary dialysis. There were four (27%) pneumonias and three (20%) cases of colonic ischemia, one requiring colectomy. One septicemia was treated with antibiotics. Overall two patients did not experience any complication, 7 patients experienced 1, 2 experienced 2, 1 experienced 3 and 1 experienced 5 complications(Table II).
Follow-up
The mean ICU/ hospital stay was 11/ 25 days. Among the four patients with endograft, one had residual type 1B endoleak distal to his thoraflex arch hybrid prosthesis. This endoleak was sealed with TEVAR 6 months later. Over a mean follow-up of 21 months, no additional death was noted, all target vessels were patent except from two IMAs (97% of patency). One patient developed visceral herniation. The four patients received a stent-graft remained free from any endoleak. (Figure 2).
DISCUSSION
Technique
Single-stemmed RVG (SSRVG) is a safe and efficient method for abdominal debranching. All target vessels but two IMAs of our series remained patent to the last follow up. IMA is notoriously known to be provided with backflow from collaterals stemming from the SMA or the hypogastric arteries. We retrospectively believe that repair of the two occluded IMAs was probably redundant. To our knowledge, the use of a SSRVG to revascularize all four (LRA, RRA, SMA and CT) even five (IMA) visceral arteries is original. In the literature, visceral rerouting techniques include customized Y-grafts, reversed bifurcated grafts, trifurcated grafts or even four-vessel visceral debranching graft as previously described by others5,7-11. All these techniques inherit the disadvantages of the “octopus”technique: several retrograde small conduits with questionable hemodynamics, complex routing and a definite risk of visceral fistula formation. Our single-stemmed set-up has on the contrary the following advantages: There is only one healthy donor artery, only one proximal anastomosis between two prosthetic grafts and only one main conduit with a patency maintained by the united flow to all target vessels. The graft is short and large while optimizing its room in the abdomen and well-protected against a visceral fistula behind the LRV and the pancreas. We indeed had no visceral fistula in the series. Finally, after a learning curve, this technique is considered easier than the complex multiple-branches technique. All our major target vessels (IMA excluded) remained patent to the last follow-up, comparing favorably with patency rates ranging between 90-97% reported elsewhere12,13,8 . To our opinion, this is the result of the hemodynamic optimization brought by the single-stem concept: a high flow in a large conduit. This provides our solution with the lowest possible hemodynamic impedance and is supported by a recent computational fluid model work14. In this work, Yuan and coll. showed that, from a hemodynamic standpoint, the use of CIA rather than aorta for the inflow site, leads to a dramatic decrease in flow to the visceral organs. Moreover, their hemodynamic model favored the use of less branches to perfuse visceral organs. In our single stem technique, should moreover one target-vessel occlude, this would not impact the patency of the others. Although all our cases have been performed through a transperitoneal approach, the technique is feasible through a retroperitoneal approach. However, such a retroperitoneal approach limits the access to the distal SMA and the distal RRA, even in the pre-renal plane. Moreover, access to the SMA and the RRA through a retroperitoneal route necessitates a thorough and extensive mobilization of the visceral segment of the aorta which should be avoided in this cohort of patients considered for debranching.
Our mortality and morbidity rates are high underlining the severity of the treated disease and the comorbidities of the patients selected for debranching. Of note, the three deaths occurred in the TAA group of patients, who were clearly the most fragile and in one case operated on an emergency setting. Mortality rates range between 10% to 15%12,13,5 in the literature, but is highly associated with patients comorbidity. However, we would like to underline the fact that none of our complications was due to occlusion of a target-vessel or the presented technique in general.
Despite limitations due to a small number of patients and its retrospective nature, the present work describes a promising technique of abdominal debranching. Comparison of debranching or hybrid surgery between open repair and totally endovascular alternatives is clearly outside of our focus. The high proportion of AD in this series shows the usefulness of our technique in this subgroup of complex aortic patients. Moreover, and as shown above, SSRVG and open aortic fenestration are highly compatible, offering an efficient solution in challenging cases.
Of course, our conclusions deserve confirmation among larger cohorts with a longer follow-up. They stress the importance of maintaining a high degree of open surgical competence among vascular surgeons, even in the endovascular era.
References
- Maurel B, Mastracci TM, Spear R, Hertault A, Azzaoui R, Sobocinski J, et al. Branched and fenestrated options to treat aortic arch aneurysms. J Cardiovasc Surg (Torino) 2016;57(5):686-97.
- Kudo T, Kuratani T, Shimamura K, Sawa Y. Total endovascular aortic repairs using branched devices for arch and thoracoabdominal aneurysms. Gen Thorac Cardiovasc Surg 2020. Doi: 10.1007/s11748-020-01411-5
- Georg Y, Schwein A, Lejay A, Lucereau B, Thaveau F, Chakfe N. Systematic cervical approach for endovascular treatment of thoracic aortic diseases with debranching. J Cardiovasc Surg (Torino) 2016;57(4):540-2.
- Bellamkonda KS, Yousef S, Nassiri N, Dardik A, Guzman RJ, Geirsson A, et al. Trends and Outcomes of TEVAR with Open Concomitant Cervical Debranching. J Vasc Surg 2020. Doi: 10.1016/j.jvs.2020.07.103.
- Drinkwater SL, Böckler D, Eckstein H, Cheshire NJW, Ko telis D, Wolf O, et al. The visceral hybrid repair of
thoraco-abdominal aortic aneurysms–a collaborative approach. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg 2009;38(5):578-85. Doi: 10.1016/j.ejvs.2009.07.002. - Shahverdyan R, Gawenda M, Brunkwall J. Five-year patency rates of renal and visceral bypasses after abdominal debranching for thoraco abdominal aortic aneurysms. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg 2013;45(6):648-56. Doi: 10.1016/j.ejvs.2013.03.012.
- Quiñones-Baldrich WJ, Panetta TF, Vescera CL, Kashyap VS. Repair of type IV thoracoabdominal aneurysm with a combined endovascular and surgical approach. J Vasc Surg 1999;30(3):555-60. Doi: 10.1016/s0741-5214(99)70084-4
- G.S. Oderich, G.A. Escobar, P. Gloviczki, T.C. Bower, B. Mendes. Hybrid Repair Using Visceral Debranching and Aortic Stent Grafts to Treat Complex Aortic Aneurysms. Endovascular Aortic Repair. Springer; n.d.
- Ham SW, Chong T, Moos J, Rowe VL, Cohen RG, Cunningham MJ, et al. Arch and visceral/renal debranching combined with endovascular repair for thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg 2011;54(1):30 40; discussion 40-41. Doi: 10.1016/j.jvs.2010.12.033.
- shomba Y, Melissano G, Logaldo D, Rinaldi E, Bertoglio L, Civilini E, et al. Clinical outcomes of hybrid repair for thoracoabdominal aortic aneurysms. Ann Cardiothorac Surg 2012;1(3):293-303. Doi: 10.3978/j.issn.2225-319X.2012.07.15.
- Hughes GC, Barfield ME, Shah AA, Williams JB, Kuchibhatla M, Hanna JM, et al. Staged total abdominal debranching and thoracic endovascular aortic repair for thoracoabdominal aneurysm. J Vasc Surg 2012;56(3):621-9. Doi: 10.1016/j.jvs.2011.11.149.
- akoyiannis C, Kalles V, Economopoulos K, Georgopoulos S, Tsigris C, Papalambros E. Hybrid procedures in the treatment of thoracoabdominal aortic aneurysms: a systematic review. J Endovasc Ther Off J Int Soc Endovasc Spec 2009;16(4):443-50. Doi: 10.1583/1545-1550-16.4.443.
- Moulakakis KG, Mylonas SN, Avgerinos ED, Kakisis JD, Brunkwall J, Liapis CD. Hybrid open endovascular technique for aortic thoracoabdominal pathologies. Circulation 2011;124(24):2670-80. Doi: 10.1161/CIRCULATION- AHA.111.041582.
- Yuan D, Wen J, Peng L, Zhao J, Zheng T. Precise plan of hybrid treatment for thoracoabdominal aortic aneurysm: Hemodynamics of retrograde reconstruction visceral arteries from the iliac artery. PloS One 2018;13(10):e0205679. Doi: 10.1371/journal.pone.0205679.
ACKNOWLEDGEMENT
We thank Dr B Feito for his time and help for the drawings.