Perioperative Management of Antiplatelet and Anticoagulation Therapy in Vascular Surgery

| Available Online: | September, 2023 |
| Page: | 52-59 |
Author for correspondence:
Slobodan Tanaskovic, MD, PhD
Vascular Surgery Clinic, Dedinje Cardiovascular Institute, Heroja Milana Tepica 1, 11000 Beograd
Email: rslobex@yahoo.com
doi: 10.59037/hjves.v5i2.47
ISSN: 2732-7175 / 2023 Hellenic Society of Vascular and Endovascular Surgery Published by Rotonda Publications
All rights reserved. https://www.heljves.com
Slobodan Tanaskovic, MD, PhD 1,2, Jovan Petrovic, MD 1,Milorad Sevkovic, MD 1, Bojan Vucurevic, MD 1, Andriana Bucic, MD 1, Danica Bajcetic, MD1, Nenad Ilijevski, MD, PhD 1,2, Petar Dabic, MD, PhD 1
1 Vascular Surgery Clinic, Dedinje Cardiovascular Institute
2 University of Belgrade – Faculty of Medicine
The authors declare that there is no conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector
Keywords: anticoagulant therapy, antiplatelet therapy, vascular surgery, perioperative period
Abstract
Full Text
References
Images
Abstract
Abstract:
Treatment of patients taking anticoagulant therapy (ACT) and antiplatelet therapy (APT) is a daily challenge for doctors of all specialities, and a special problem is the adequate care of these patients in the immediate perioperative period during vascular surgical procedures. This paper presents the current findings and recommendations on the perioperative use of ACT and APT and considerations of therapeutic modalities in frequent clinical cases of vascular patients. An overview of the most commonly used anticoagulant and antiplatelet drugs in clinical practice is also presented. Vascular surgical patients represent a population of patients in whom platelet coagulation and aggregation mechanism are dysregulated in many cases. There is still no broad consensus and unequivocal evidence that can direct the physician towards the right modality of therapy. The final decision rests with the physician, who should, based on the individual assessment of each patient, determine the risk and thus determine the modality of anticoagulant and antiplatelet therapy.
Full Text
1. INTRODUCTION
Treatment of patients taking anticoagulant therapy (ACT) and/ or antiplatelet therapy (APT) is a daily challenge for doctors of all specialities, and the adequate care of these patients in the immediate perioperative period during vascular surgical procedures represents a unique problem 1. Discontinuation of therapy may increase the risk of thromboembolic events during and after surgery, while continuing therapy may increase the risk of bleeding during surgery and cause several other adverse events in the immediate postoperative period2,3. Therefore, the question arises in which situations the therapy should be stopped, and when it is necessary to extend the use of anticoagulant and antiplatelet drugs in the immediate perioperative period.
In planning elective operative procedures, the surgeon must address whether AK therapy should be paused, continued or bridged with heparin or low-molecular-weight heparins (LMWHs). This decision is influenced by several factors, such as various patient characteristics (kidney function, indications or ACT, age, history of bleeding or thromboembolic events), as well as surgical factors (risk of perioperative bleeding etc.) 2.
2. AETIOLOGY AND EPIDEMIOLOGY
Atrial fibrillation (AF), deep vein thrombosis (DVT) and pulmo- nary embolism (PE) are the main indications for prescribing ACT. AF is the most common long-term cardiac arrhythmia in adults 4. In the US alone, between 3 and 5 million people suffer from AF, and estimates are that this number will increase to 8 million by 2050, while the number of patients with AF in Europe could be almost 18 million by 2060 5,6 . The trend of increasing prevalence and incidence of AF will continue in the next 30 years, especially in countries with a medium sociode- mographic index 5 .
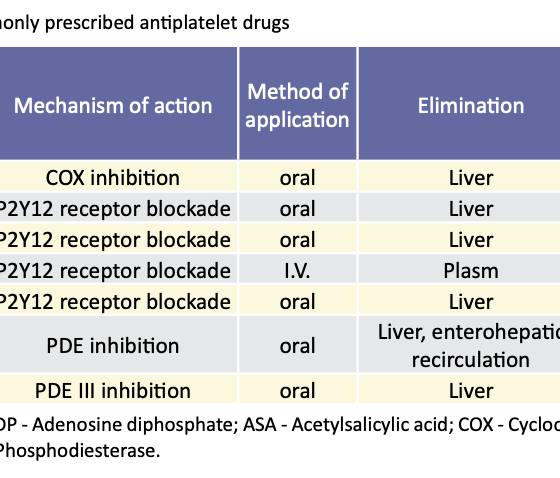
PT is the basis of the treatment of patients with cardiovascular diseases. Patients undergoing percutaneous coronary intervention (PCI) are usually on dual antiplatelet therapy (DAPT), as well as patients with a previous history of stroke, aortocoronary bypass, essential thrombocytosis, etc1 . Acetylsalicylic acid (ASA) is one of the most commonly prescribed drugs in the world and the most commonly prescribed anti- platelet drug in the treatment of cardiovascular and cerebro- vascular diseases 7. More than 30% of people over the age of 50 in the USA have been reported to use ASA to prevent adverse cardiovascular events 8 . DAPT, on the other hand, involves combining ASA with a P2Y12 inhibitor (clopidogrel, prasugrel, ticagrelor)9. Based on estimates from 2015, between 1.4 and 2.2 million patients annually have an indication for DAPT after PCI or myocardial infarction (MI) worldwide. Base on more than 35 randomized clinical trials, which included more than 225 thousand patients, it was concluded that DAPT is among the most common treatment options in the field of cardiovascular medicine 9 . An overview of the most commonly used antiplatelet drugs is given in Table 1.
3. PHARMACOLOGY
3.1. Antiplatelet drug
Small doses of orally administered acetylsalicylic acid irreversibly inhibit platelet cyclooxygenase (COX) 1 and 2 (significantly more inhibits the COX-1 isoenzyme), thereby preventing the enzymatic creation of thromboxane A2, a strong activator of platelet aggregation and vasoconstriction7,11 . Although a daily dose of 30 mg is sufficient to completely inhibit COX-1 in platelets, a daily dose of 75-150 mg for long-term prevention and a daily dose of 150-325 mg for rapid and complete inhibition of platelet aggregation is recommended in cardiovascular patients12.
3.1.2. Nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAID), unlike ASA, reversibly inhibit COX-1 and COX-2 isoenzymes. Their effect on platelet function is short-term and normalizes within three days. However, this can vary between different drugs in the group. For short-acting drugs such as ibuprofen, diclofenac and indomethacin, 50% of platelet function is restored by 6 hours after the last dose and normalizes after 24 hours 7,10,12 .
3.1.3. Thienopyridines (Clopidogrel and Prasugrel)
Drugs from this group achieve their antiplatelet effect by irreversibly inhibiting the P2Y12 receptor on platelets, thus preventing the binding of adenosine diphosphate (ADP) and platelet activation mediated by it7,10 . Clopidogrel is not used as the first-choice drug in monotherapy unless there is a proven hypersensitivity of the patient to ASA10,12 . However, new studies show the advantage of clopidogrel monotherapy compared with ASA in reducing mortality and morbidity in patients after PCI with drug-eluting stents (DES)13 . According to numerous studies, prasugrel has been shown to be a superior drug compared to clopidogrel in terms of inhibition of aggregation, but not in terms of the risk of bleeding, which is higher with this drug compared to clopidogrel 10,14 . Due to the irreversible mechanism of action, it is recommended to discontinue these drugs 5 to 7 days before elective non-cardiac surgery1,15 .
3.1.4. Ticagrelor and cangrelo
This group consists of relatively new drugs with different mechanisms of action compared to the drugs known so far. Ticagrelor is an orally administered reversibly binding agent that selectively and potently blocks ADP-induced P2Y12 receptor signalling 7,10 . Cangrelor is a drug that is administered intravenously, it is a strong and directly acting platelet ADP inhibitor, with a rapid onset of action. Due to the interaction of metabolites of thienopyridine antiplatelet drugs with cangrelor, caution is necessary when transferring patients to clopidogrel or prasugrel therapy; this should be done only after the cangrelor infusion has been stopped to ensure P2Y12 inhibition7 .
3.1.5. Glycoprotein IIb/IIIa inhibitors
Drugs that directly block the glycoprotein (GP) IIb/IIIa receptor have been shown to be more effective in inhibiting in vivo platelet aggregation than ASA and clopidogrel and are associated with superior early clinical outcomes in patients with coronary artery disease and those undergoing PCI. Three drugs from this group, that have been approved by the Food and Drug Administration (FDA) are abciximab, tirofiban and eptifibatide7 .
3.2. Anticoagulant drugs
3.2.1. Vitamin K antagonists (coumarins
The most commonly used drug from this group is warfarin, which has been on the market for over 50 years 16. Its mechanism of action is based on the inhibition of the enzyme responsible for the conversion of vitamin K from oxidized to the reduced form. Vitamin K, on the other hand, is a necessary cofactor in the carboxylation reaction that leads to the activation of coagulation factors II, VII, IX and X17. In practice, warfarin is a drug whose therapeutic dose is difficult to achieve, due to a large number of pharmacological interactions and genetic variations that can affect its metabolism. 17
3.2.2. Direct-acting oral anticoagulants (DOACs)
he basic mechanism of action of these drugs, which include rivaroxaban, apixaban and edoxaban, is direct inhibition of factor Xa. In addition to them, this group of drugs also includes a direct thrombin inhibitor, dabigatran 18. The advantages of prescribing DOACs are their short half-life and quick onset of action, which allows for the easier interruption and resumption of therapy after the operative procedure and does not require monitoring of the international normalized ratio (INR), which is an advantage compared to warfarin 19 . However, these drugs accumulate in patients with impaired renal function, there are no widely available tests to monitor their anticoagulant activity, and there are no widely available specific antidotes for neutralization in case of overdose and/or severe bleeding 18. Only idarucizumab is currently available as an antidote to dabigatran. While for other DOACs, the antidote ciraparantag (aripazine) is in the final phase of testing. Adnexanet alfa is used as an antidote in the case of rivaroxaban and apixaban overdose, and the results of randomized studies for this drug are expected in the coming years
3.2.3. Unfractionated heparin
By binding to antithrombin III (AT-III), the anticoagulant effect of heparin is realized. The complex formed by heparin and AT- III leads to irreversible inhibition of thrombin, as well as factor Xa 20. Anticoagulant response to heparin administration is monitored using activated partial thromboplastin time (aPTT). Major complications of unfractionated heparin therapy include bleeding (major bleeding, 0-7%; fatal bleeding, 0-3%) and heparin-induced thrombocytopenia (HIT, 1-5%) 19.
3.2.4. Low Molecular Weight Heparins
MWHs have higher bioavailability after subcutaneous injection, a renal clearance that is independent of dose, and a longer half-life (about 20h) compared to unfractionated heparin. Because of their predictable dose response, laboratory monitoring of anticoagulant activity is usually not necessary. Anti-Xa monitoring is an option in high-risk patient populations (renal insufficiency, obesity, pregnancy) where dose adjustments may be necessary19 . LMWHs are the drugs most often used in bridging anticoagulant therapy 19.
3.2.5. Fondaparinux
Fondaparinux (pentasaccharide) achieves its effect by indi-rectly inhibiting factor Xa, binding to antithrombin, thereby potentiating its activity. The anticoagulant activity lasts up to 4 days after the last dose of the drug in a person with preserved kidney function19 . A comparison of certain pharmacological properties of heparin and its derivatives is given in Table 2.
4. RISK ASSESSMEN
The approach recommended by several guidelines is based on four items intended to guide the physician through cases of elective surgery1,15,21,22
4.1. Thromboembolic risk assessment
he three factors with the greatest contribution to the risk for a thromboembolic event are atrial fibrillation, an artificial heart valve, and a previous thromboembolic event. The CHA2 DS 2 VAS c score is used to assess the risk of atrial fibrillation 23. Location, type of valve, number of prosthetic valves, and other cardiac risk factors are used in risk stratification of patients with prosthetic valves. As for thromboembolism, the time after the episode and the risk of recurrence will d etermine the degree of risk. Venous thromboembolism includes DVT and PE and can be classified as provoked (carries a higher risk of recurrence) when a causative event can be identified, or unprovoked when a cause cannot be identified24 . An exam- ple of induced VTE is a patient with persistent risk factors such as congestive heart failure, hereditary thrombophilia or paraneoplastic syndrome1 . The degree of risk is given in Table 3 .
4.2. Bleeding risk assessment
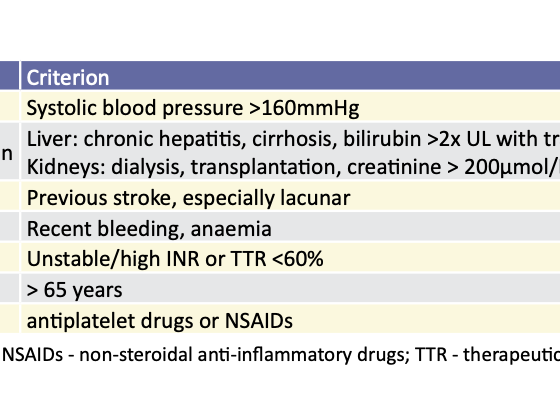
When assessing the risk of bleeding, it is very important to consider the type of surgical procedure the patient is to undergo. Procedural bleeding risk can be divided into low risk (0-2% two-day bleeding risk) or high risk (2-4% two-day bleeding risk)1 . Vascular surgical procedures belong to the series of procedures in which the risk of acute coagulopathies and large blood loss is considerable, and in these cases, additional caution is necessary25,26. In addition, other characteristics of the patient should be taken into account. The HAS-BLED score is used for risk assessment (Table 4) 27. Each positive item earns 1 point, and a HAS-BLED score > 3 indicates a high risk of bleeding. 1
Perioperative Management of Antiplatelet and Anticoagulation Therapy in Vascular Surgery 55 4.3. When (not) to stop therapy
The basic rule in the decision to stop or continue ACT or APT is the assessment of the benefit that the patient would have to depend on the decision. Patients at increased risk for bleeding would benefit from discontinuation of therapy, while patients at increased risk for thromboembolism would benefit from “bridging” therapy or the shortest possible period without ACT. Some of the scenarios often encountered in vascular surgery are given below.
4.3.1. Venous Thromboembolism (VTE)
In patients with a recent episode of VTE (< 3 months, especially < 1 month) the risk of recurrence can be up to 40% depending on risk factors. “Personalization” of risk factor assessment could optimize the benefit-risk ratio by reliably identifying patients at lower or higher risk of recurrent VTE and deciding whether to continue or discontinue ACT28. Numerous scoring systems can be used for this purpose, but what the latest European Society of Cardiology (ESC) guide points out is that in light of the increasing use of DOAC, these scoring systems must be revised 29. Current recommendations for patients on warfarin therapy for VTE as the only indication for AK therapy are that bridging LMWH therapy is not necessary, and warfarin should be stopped at least 5 days before the procedure. This does not exclude the possibility of using prophylactic dos- s of LMWH in the perioperative period. Perioperative bridging therapy is also recommended for patients with a high risk of VTE 25 . New guidelines dealing with the continuation of warfarin therapy after a surgical procedure recommend continuation of therapy within 24 hours of the procedure. It is also advised that resumption of therapy should be started at the dose the patient used before discontinuation, even in procedures with a high risk of bleeding, with the simultaneous use of LMWH until therapeutic INR values are achieved30 .
4.3.2. Coronary stenting
n the clinical practice of vascular surgeons, patients with previously implanted coronary stents are often encountered. It is estimated that between 15% and 20% of patients require non-cardiac surgery up to 2 years after coronary stent implantation 30. It is advised that in patients who have had a stent implanted less than 3 months before a vascular procedure, DAPT should be continued in case of a procedure with a low bleeding risk or P2Y12 inhibitor should be excluded, and ASA therapy should be continued in case of major surgery. If coronary stents have been placed between 3 and 12 months, it is possible to stop P2Y12 inhibitors and continue ASA therapy. Clear recommendations regarding the return of patients to DAPT have not been given, but it is considered that in the case when patients are not excluded from therapy with ASA, it is possible to return to therapy with clopidogrel up to 24-72 hours post-procedural in the loading dose 25 .
However, each case should be approached individually and a decision made following the patient’s characteristics and illness.
A practical classification of vascular procedures concerning the risk of bleeding and thromboembolic events in patients after coronary stenting was given by Rossini et al.30. It should be emphasized that the overall risk of thromboembolic events was assessed based on several parameters such as the type of stent, clinical and angiographic characteristics and time from PCI procedure to surgery.
4.3.3. Atrial fibrillation
The presence of atrial fibrillation in patients with the peripheral arterial occlusive disease (POAB) is not uncommon. Some studies report that about 20% of patients with atrial fibrilla- tion have a pedobrachial index < 0.9031. Also, it is estimated that 5-10% of patients after a vascular procedure develop postoperative atrial fibrillation23 . The recommendations are that in the perioperative period, patients suffering from atrial fibrillation should be treated in such a way that, based on an individual assessment, as well as different scoring systems (HAS-BLED, CHA₂DS₂-VASc), the use of different AK modalities or “bridging” therapy should be decided, in depending on the risk of a thromboembolic event or bleeding 23 .
4.3.4 Triple therapy
It should be noted that around 10% of patients with recent PCI have AF and others could have VTE, so choosing the right antithrombotic regimen can be a challenge, especially in the setting of a potential vascular surgical procedure. Usually, the use of triple therapy (DAPT and anticoagulant) is not recommended for most patients due to an increased risk of bleeding. However, there are cases in which triple therapy is needed to achieve the best outcome for the patients, but currently, there are no clear guidelines for the management of triple therapy in the perioperative period. It is suggested that, for patients presenting with AF appropriate for an OAC who have a prior history of cerebrovascular disease and are currently receiving APT who have undergone recent carotid endarterectomy, stopping all APT and treating with an ACT alone (DOAC preferred) when considered safe from the risk of postoperative bleeding, typically 3 to 14 days after surgery 32 . It should be noted that in these circumstances continuing only ASA and bridging therapy could be the safest option, but more evidence is needed in this regard.
Table 5provides an overview of vascular procedures concerning the risk of bleeding, with the suggestion of using antiplatelet and anticoagulant therapy in the perioperative period depending on the risk of a thromboembolic event.
However, a clinical assessment of stopping or continuing ACT or APT is imperative, because there are no scores or calculators that would directly and unambiguously classify the patient into one of the categories.
5. BRIDGING THERAPY
The bridging ACT consists of replacing a long-acting anticoagulant (warfarin) in the perioperative period with a short-acting anticoagulant (LMVH) when the INR is below the therapeutic level, to limit the time of subtherapeutic levels of anticoagulation and reduce the risk of thromboembolism 25 . Despite growing evidence of limited or even no benefit to bridging therapy, it is still used on a case-by-case basis. Also, few studies and guides are dealing with this type of therapy in vascular patients. In a study by Siegal et al.33 it was shown that there is no statistically significant difference concerning the risk of a thromboembolic event in patients who were on “bridging” therapy and those who only stopped warfarin. On the other hand, the risk of bleeding was up to 3 times higher in the group of patients on bridging therapy. However, this meta-analysis has been criticized a lot because of the heterogeneity of the studies included in it. The latest guidelines also advise against bridging therapy. Douketis et al. 25 state that in cases where it is necessary to stop warfarin therapy in patients with mechanical valves, atrial fibrillation or when the use of warfarin is indicated only because of VTE, bridging therapy with LMWH is not necessary, however, the level of evidence for this recommendation is low, and the authors themselves point out that despite this new knowledge, uncertainty remains regarding best practice for most issues of perioperative use of ACT. It is emphasized that an analysis of each case is necessary and that patients with a high risk for a thromboembolic event ( Table 3) should receive adequate bridging therapy. Douketis et al. 25 state that it is not necessary to introduce bridging therapy in patients using DOAC due to the known pharmacokinetic properties of the drugs (Table 6).
CONCLUSION
Vascular surgical patients represent a population of complex patients in whom platelet coagulation and aggregation mechanism are dysregulated in many cases. Although there are currently numerous studies and guides that directly or indirectly deal with the perioperative use of anticoagulant and antiplatelet therapy, there is no broad consensus and unequivocal evidence that can guide the physician towards the right modality of therapy, especially in complex vascular patients. The final decision rests with the doctor, who should, based on the individual assessment of each patient, determine the risk and thus determine the modality of anticoagulant and/or antiplatelet therapy.
References
- Gutierrez JJP, Rocuts KR. Perioperative Anticoagulation Management. StatPearls. Treasure Island: StatPearls Publishing; 2022.
- Wagner J, Lock JF, Kastner C, Klein I, Krajinovic K, Löb S, et al. Perioperative management of anticoagulant therapy. Innov Surg Sci. 2019;4:144-51.
- Briete LD, Towers WF, Bone R, Nair R, Steck M, Cutshall BT, et al. Perioperative Anticoagulation Management. Crit Care Nurs Q. 2022;45:119-31.
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Sta- tistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56-528.
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217-21.
- ulkifly H, Lip GYH, Lane DA. Epidemiology of atrial fibrillation. Int J Clin Pract. 2018;72(3):e13070
- Kalra K, Franzese CJ, Gesheff MG, Lev EI, Pandya S, Bliden KP, et al. Pharmacology of antiplatelet agents. Curr Atheroscler Rep. 2013;15(12).
- Boakye E, Uddin SMI, Obisesan OH, Osei AD, Dzaye O, Sharma G, et al. Aspirin for cardiovascular disease prevention among adults in the United States: Trends, prevalence, and participant characteristics associated with use. Am J Prev Cardiol. 2021;8:100256.
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-54.
- Hall R, Mazer CD. Antiplatelet drugs: A review of their pharmacology and management in the perioperative period. Anesth Analg. 2011;112(2):292-318.
- Nagelschmitz J, Blunck M, Kraetzschmar J, Ludwig M, Wensing G, Hohlfeld T. Pharmacokinetics and pharmacodynamics of acetylsalicylic acid after intravenous and oral administration to healthy volunteers. Clin Pharmacol. 2014;6(1):51-9.
- Gurbel PA, Tantry US. Combination antithrombotic therapies. Circulation. 2010;121:569-83.
- Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. The Lancet. 2021;397(10293):2487-96.
- Angiolillo DJ, Bhatt DL, Gurbel PA, Jennings LK. Advances in Antiplatelet Therapy: Agents in Clinical Development. Am J Cardiol. 2009;103(3 SUPPL.):40A-51A
- Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, de Hert S, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-431.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Vascular Medicine Periprocedural Heparin Bridging in Patients Receiving Vitamin K Antagonists Systematic Review and Meta-Analysis of Bleeding and Thromboembolic Rates. Circulation. 2012;126:1630-9.
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):160S-198S.
- Gómez-Outes A, Suárez-Gea ML, Lecumberri R, Terlei- ra-Fernández AI, Vargas-Castrillón E. Direct-acting oral anticoagulants: pharmacology, indications, management, and future perspectives. Eur J Haematol. 2015;95(5):389- 404.
- Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants: A Review of the Pharmacology, Dosing, and Complications. Curr Emerg Hosp Med Rep. 2013;1(2):83-97.
- Mulloy B, Hogwood J, Gray E, Lever R, Page CP. Pharmacology of Heparin and Related Drugs. Pharmacol Rev. 2016;68(1):76-141.
- Fleisher L, Fleischmann K, Auerbach A, Barnason S, Beckman J, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e278-e333.
- Naylor A, Rantner B, Ancetti S, de Borst G, de Carlo M, Halliday A, et al. European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 2022;20.
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax, JJ, Blomström-Lundqvist, C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42:373-498.
- Fahrni J, Husmann M, Gretener SB, Keo HH. Assessing the risk of recurrent venous thromboembolism – a practical approach. Vasc Health Risk Manag. 2015;11:451-9.
- Douketis JD, Spyropoulos AC, Murad MH, Arcelus JI, Dager WE, Dunn AS, et al. Perioperative Management of Antithrombotic Therapy. Chest. 2022;162(5):e207-e43
- Chee YE, Liu SE, Irwin MG. Management of bleeding in vascular surgery. Br J Anaesth. 2016;117(suppl2):ii85-94.
- Zhu W, He W, Guo L, Wang X, Hong K. The HAS-BLED Score for Predicting Major Bleeding Risk in Anticoagulated Patients with Atrial Fibrillation: A Systematic Review and Meta-analysis. Clin Cardiol. 2015;38(9):555.
- Áinle FN, Kevane B. Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)? Blood Adv. 2020;4(21):5595.
- Konstantinides S v., Meyer G, Bueno H, Galié N, Gibbs JSR,geno W, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603.
- Rossini R, Tarantini G, Musumeci G, Masiero G, Barbato E, Calabrò P, et al. A Multidisciplinary Approach on the Perioperative Antithrombotic Management of Patients with Coronary Stents Undergoing Surgery: Surgery After Stenting 2. JACC Cardiovasc Interv. 2018;11(5):417-34.
- Violi F, Daví G, Hiatt W, Lip GYH, Corazza GR, Perticone F, et al. Prevalence of Peripheral Artery Disease by Abnormal Ankle-Brachial Index in Atrial Fibrillation: Implications for Risk and Therapy. J Am Coll Cardiol. 2013;62(23):2255-6.
- Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, et al. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients with Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(5):629-58.
- Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-9.